Bromination Of Acetanilide Balanced Equation
Benzene & Derivatives
Substitution Reactions of Benzene and Other Aromatic Compounds
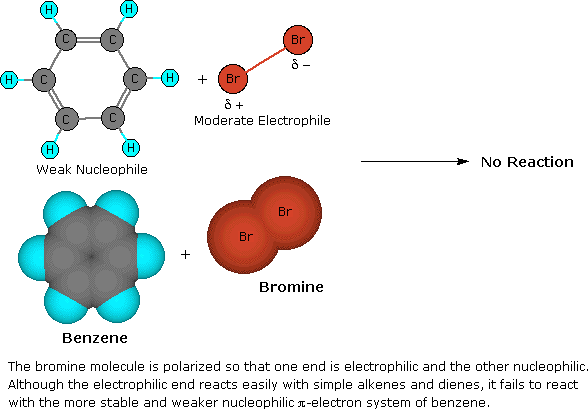
The remarkable stability of the unsaturated hydrocarbon benzene has been discussed in an earlier chapter. The chemical reactivity of benzene contrasts with that of the alkenes in that substitution reactions occur in preference to improver reactions, as illustrated in the following diagram (some comparable reactions of cyclohexene are shown in the green box).
Many other substitution reactions of benzene have been observed, the 5 most useful are listed below (chlorination and bromination are the near common halogenation reactions). Since the reagents and conditions employed in these reactions are electrophilic, these reactions are commonly referred to equally Electrophilic Aromatic Substitution. The catalysts and co-reagents serve to generate the strong electrophilic species needed to effect the initial step of the exchange. The specific electrophile believed to function in each type of reaction is listed in the right hand cavalcade.
| Reaction Type | Typical Equation | Electrophile Due east(+) | |||
|---|---|---|---|---|---|
| Halogenation: | C6H6 | + Cltwo & heat FeCl3 catalyst | —— > | C6H5Cl + HCl Chlorobenzene | Cl(+) or Br(+) |
| Nitration: | C6H6 | + HNO3 & heat H2SO4 catalyst | —— > | C6H5NO2 + HiiO Nitrobenzene | NOtwo (+) |
| Sulfonation: | C6H6 | + H2SO4 + So3 & heat | —— > | C6H5SO3H + HiiO Benzenesulfonic acid | And theniiiH(+) |
| Alkylation: Friedel-Crafts | Chalf-dozenH6 | + R-Cl & estrus AlClthree goad | —— > | C6H5-R + HCl An Arene | R(+) |
| Acylation: Friedel-Crafts | Chalf-dozenH6 | + RCOCl & rut AlCl3 goad | —— > | Chalf-dozenH5COR + HCl An Aryl Ketone | RCO(+) |
1. A Mechanism for Electrophilic Substitution Reactions of Benzene
A two-footstep mechanism has been proposed for these electrophilic commutation reactions. In the first, slow or rate-determining, stride the electrophile forms a sigma-bond to the benzene band, generating a positively charged benzenonium intermediate. In the 2d, fast step, a proton is removed from this intermediate, yielding a substituted benzene band. The following four-part illustration shows this mechanism for the bromination reaction. Also, an animated diagram may be viewed.
Bromination of Benzene - An Example of Electrophilic Effluvious Exchange
 |  |
| There are iv stages to this slide evidence. These may be viewed repeatedly by continued clicking of the "Next Slide" button. | |
This machinery for electrophilic aromatic substitution should be considered in context with other mechanisms involving carbocation intermediates. These include SouthwardN1 and E1 reactions of alkyl halides, and Brønsted acid addition reactions to alkenes.
1. The cation may bond to a nucleophile to give a substitution or add-on product.
ii. The cation may transfer a proton to a base of operations, giving a double bond product.
3. The cation may rearrange to a more than stable carbocation, and and then react by fashion #1 or #ii.
SN1 and E1 reactions are corresponding examples of the first two modes of reaction. The 2d footstep of alkene addition reactions gain by the first mode, and any of these 3 reactions may exhibit molecular rearrangement if an initial unstable carbocation is formed. The carbocation intermediate in electrophilic aromatic exchange (the benzenonium ion) is stabilized past charge delocalization (resonance) then it is not discipline to rearrangement. In principle it could react past either mode 1 or 2, just the energetic reward of reforming an aromatic ring leads to exclusive reaction by mode ii (ie. proton loss).
2. Ring Substitution Reactions of Benzene Derivatives
When substituted benzene compounds undergo electrophilic commutation reactions of the kind discussed to a higher place, ii related features must exist considered:
I. The start is the relative reactivity of the compound compared with benzene itself. Experiments have shown that substituents on a benzene ring can influence reactivity in a profound manner. For example, a hydroxy or methoxy substituent increases the rate of electrophilic substitution nigh x 1000 fold, as illustrated by the case of anisole in the virtual sit-in (higher up). In contrast, a nitro substituent decreases the ring's reactivity by roughly a million. This activation or deactivation of the benzene band toward electrophilic substitution may exist correlated with the electron donating or electron withdrawing influence of the substituents, as measured past molecular dipole moments. In the post-obit diagram we see that electron donating substituents (blue dipoles) activate the benzene ring toward electrophilic set on, and electron withdrawing substituents (red dipoles) deactivate the ring (make it less reactive to electrophilic attack).
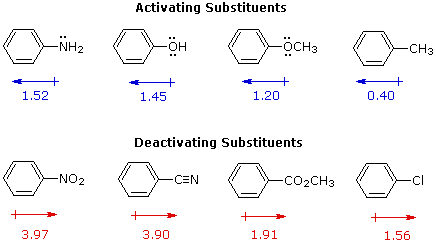
The influence a substituent exerts on the reactivity of a benzene ring may be explained past the interaction of two effects:
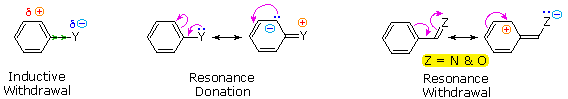
The starting time is the anterior upshot of the substituent. Most elements other than metals and carbon have a significantly greater electronegativity than hydrogen. Consequently, substituents in which nitrogen, oxygen and halogen atoms form sigma-bonds to the aromatic ring exert an anterior electron withdrawal, which deactivates the ring (left-manus diagram below).
The second upshot is the result of conjugation of a substituent function with the aromatic ring. This conjugative interaction facilitates electron pair donation or withdrawal, to or from the benzene band, in a manner dissimilar from the inductive shift. If the atom bonded to the ring has 1 or more non-bonding valence shell electron pairs, equally do nitrogen, oxygen and the halogens, electrons may menstruum into the aromatic band by p-π conjugation (resonance), every bit in the middle diagram. Finally, polar double and triple bonds conjugated with the benzene band may withdraw electrons, as in the right-hand diagram. Note that in the resonance examples all the contributors are not shown. In both cases the accuse distribution in the benzene band is greatest at sites ortho and para to the substituent.
In the case of the nitrogen and oxygen activating groups displayed in the meridian row of the previous diagram, electron donation past resonance dominates the inductive effect and these compounds show exceptional reactivity in electrophilic substitution reactions. Although element of group vii atoms have non-bonding valence electron pairs that participate in p-π conjugation, their potent inductive result predominates, and compounds such as chlorobenzene are less reactive than benzene. The three examples on the left of the bottom row (in the aforementioned diagram) are examples of electron withdrawal by conjugation to polar double or triple bonds, and in these cases the anterior event farther enhances the deactivation of the benzene ring. Alkyl substituents such equally methyl increment the nucleophilicity of aromatic rings in the same fashion as they act on double bonds.
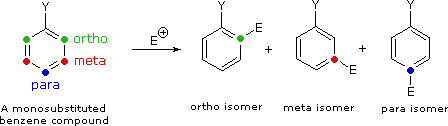
2. The second cistron that becomes important in reactions of substituted benzenes concerns the site at which electrophilic substitution occurs. Since a mono-substituted benzene band has two equivalent ortho-sites, 2 equivalent meta-sites and a unique para-site, three possible ramble isomers may be formed in such a substitution. If reaction occurs as well at all available sites, the expected statistical mixture of isomeric products would be xl% ortho, 40% meta and 20% para. Over again nosotros find that the nature of the substituent influences this product ratio in a dramatic way. Bromination of methoxybenzene (anisole) is very fast and gives mainly the para-bromo isomer, accompanied by x% of the ortho-isomer and only a trace of the meta-isomer. Bromination of nitrobenzene requires potent heating and produces the meta-bromo isomer as the main product.
Some additional examples of product isomer distribution in other electrophilic substitutions are given in the table below. It is important to note here that the reaction conditions for these commutation reactions are not the same, and must be adapted to fit the reactivity of the reactant CsixHfive-Y. The high reactivity of anisole, for example, requires that the first 2 reactions be conducted nether very mild weather condition (low temperature and piddling or no goad). The nitrobenzene reactant in the third example is very unreactive, so rather harsh reaction weather must exist used to accomplish that reaction.
| Y in C6H5–Y | Reaction | % Ortho-Product | % Meta-Production | % Para-Product |
|---|---|---|---|---|
| –O–CH3 | Nitration | 30–40 | 0–2 | threescore–seventy |
| –O–CHiii | F-C Acylation | 5–10 | 0–5 | xc–95 |
| –NOtwo | Nitration | five–8 | 90–95 | 0–five |
| –CH3 | Nitration | 55–65 | i–v | 35–45 |
| –CH3 | Sulfonation | 30–35 | 5–10 | 60–65 |
| –CH3 | F-C Acylation | ten–15 | 2–8 | 85–90 |
| –Br | Nitration | 35–45 | 0–four | 55–65 |
| –Br | Chlorination | 40–45 | 5–10 | 50–60 |
These observations, and many others similar them, have led chemists to formulate an empirical classification of the various substituent groups commonly encountered in effluvious commutation reactions. Thus, substituents that activate the benzene ring toward electrophilic attack by and large direct substitution to the ortho and para locations. With some exceptions, such every bit the halogens, deactivating substituents direct substitution to the meta location. The following tabular array summarizes this classification.
Orientation and Reactivity Effects of Ring Substituents | |||||||
|---|---|---|---|---|---|---|---|
| Activating Substituents | Deactivating Substituents | Deactivating Substituents | |||||
| –O(–) –OH –OR –OC6Hv –OCOCH3 | –NH2 –NR2 –NHCOCHiii –R –Chalf dozenHv | –NO2 –NRthree (+) –PRiii (+) –SRii (+) –SO3H –SO2R | –CO2H –CO2R –CONHii –CHO –COR –CN | –F –Cl –Br –I –CHtwoCl –CH=CHNO2 | |||
The information summarized in the higher up table is very useful for rationalizing and predicting the course of aromatic substitution reactions, simply in practice well-nigh chemists find it desirable to understand the underlying physical principles that contribute to this empirical nomenclature. We take already analyzed the activating or deactivating properties of substituents in terms of inductive and resonance furnishings, and these same factors may be used to rationalize their influence on substitution orientation.
The first thing to recognize is that the proportions of ortho, meta and para exchange in a given case reflect the relative rates of exchange at each of these sites. If we utilize the nitration of benzene as a reference, we can assign the rate of reaction at one of the carbons to be 1.0. Since in that location are vi equivalent carbons in benzene, the total charge per unit would be 6.0. If we examine the nitration of toluene, tert-butylbenzene, chlorobenzene and ethyl benzoate in the same way, we can assign relative rates to the ortho, meta and para sites in each of these compounds. These relative rates are shown (colored red) in the following illustration, and the total rate given below each structure reflects the 2 to 1 ratio of ortho and meta sites to the para position. The overall relative rates of reaction, referenced to benzene as 1.0, are calculated past dividing by vi. Clearly, the alkyl substituents activate the benzene ring in the nitration reaction, and the chlorine and ester substituents deactivate the ring.
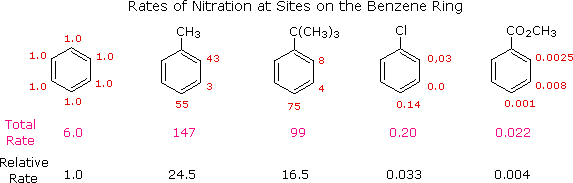
From charge per unit data of this kind, information technology is a simple matter to calculate the proportions of the three substitution isomers. Toluene gives 58.five% ortho-nitrotoluene, 37% para-nitrotoluene and only 4.5% of the meta isomer. The increased bulk of the tert-butyl group hinders attack at the ortho-sites, the overall production mixture being 16% ortho, 8% meta and 75% para-nitro product. Although chlorobenzene is much less reactive than benzene, the rate of ortho and para-substitution greatly exceeds that of meta-substitution, giving a product mixture of thirty% ortho and lxx% para-nitrochlorobenzene. Finally, the benzoic ester gave predominantly the meta-nitro production (73%) accompanied past the ortho (22%) and para (5%) isomers, as shown by the relative rates. Equivalent rate and product studies for other commutation reactions pb to similar conclusions. For example, electrophilic chlorination of toluene occurs hundreds of times faster than chlorination of benzene, only the relative rates are such that the products are 60% ortho-chlorotoluene, 39% para and 1% meta-isomers, a ratio like to that observed for nitration.
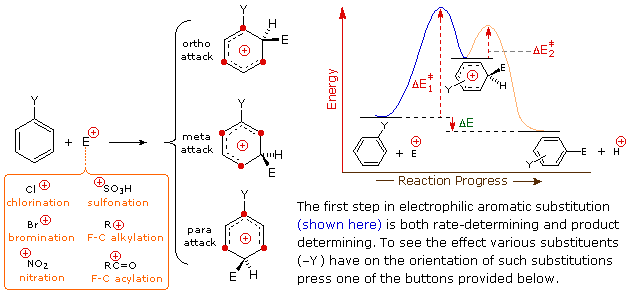
The manner in which specific substituents influence the orientation of electrophilic substitution of a benzene band is shown in the following interactive diagram. Equally noted on the opening analogy, the product-determining footstep in the substitution mechanism is the first step, which is likewise the slow or rate determining pace. It is non surprising, therefore, that in that location is a rough correlation betwixt the rate-enhancing effect of a substituent and its site directing influence. The exact influence of a given substituent is best seen by looking at its interactions with the delocalized positive accuse on the benzenonium intermediates generated by bonding to the electrophile at each of the 3 exchange sites. This tin be washed for seven representative substituents past using the selection buttons underneath the diagram.
 | |||||||
| Y– | CH3 | Cl or Br | NOtwo | RC=O | And so3H | OH | NHtwo |
|---|---|---|---|---|---|---|---|
In the case of alkyl substituents, charge stabilization is greatest when the alkyl group is bonded to 1 of the positively charged carbons of the benzenonium intermediate. This happens simply for ortho and para electrophilic attack, so such substituents favor formation of those products. Interestingly, primary alkyl substituents, peculiarly methyl, provide greater stabilization of an adjacent charge than do more substituted groups (note the greater reactivity of toluene compared with tert-butylbenzene).
Nitro (NO2), sulfonic acrid (SO3H) and carbonyl (C=O) substituents have a full or partial positive charge on the atom bonded to the aromatic ring. Structures in which like-charges are shut to each other are destabilized by charge repulsion, then these substituents inhibit ortho and para commutation more than meta exchange. Consequently, meta-products preominate when electrophilic substitution is forced to occur.
Halogen ( Ten ), OR and NR2 substituents all exert a destabilizing inductive issue on an adjacent positive charge, due to the high electronegativity of the substituent atoms. By itself, this would favor meta-substitution; however, these substituent atoms all have non-bonding valence electron pairs which serve to stabilize an side by side positive charge by pi-bonding, with resulting delocalization of charge. Consequently, all these substituents direct exchange to ortho and para sites. The balance between inductive electron withdrawal and p-π conjugation is such that the nitrogen and oxygen substituents have an overall stabilizing influence on the benzenonium intermediate and increase the charge per unit of substitution markedly; whereas halogen substituents have an overall destabilizing influence.
3. Characteristics of Specific Substitution Reactions
| Halogenation: | Chalf dozenHvi | + Clii & oestrus FeCl3 goad | —— > | C6HvCl Chlorobenzene | + HCl |
| Nitration: | C6H6 | + HNO3 & heat HiiAnd so4 goad | —— > | C6HfiveNO2 Nitrobenzene | + H2O |
| Sulfonation: | C6Hsix | + HtwoSo4 + SO3 & heat | —— > | C6H5And so3H Benzenesulfonic acid | + H2O |
| Alkylation: Friedel-Crafts | C6H6 | + R-Cl & heat AlCliii goad | —— > | C6H5-R An Arene | + HCl |
| Acylation: Friedel-Crafts | C6Hsix | + RCOCl & heat AlCl3 goad | —— > | C6HvCOR An Aryl Ketone | + HCl |
The conditions commonly used for the aromatic substitution reactions discussed hither are repeated in the table on the correct. The electrophilic reactivity of these unlike reagents varies. Nosotros find, for case, that nitration of nitrobenzene occurs smoothly at 95 ºC, giving meta-dinitrobenzene, whereas bromination of nitrobenzene (ferric catalyst) requires a temperature of 140 ºC. Besides, equally noted earlier, toluene undergoes nitration about 25 times faster than benzene, but chlorination of toluene is over 500 times faster than that of benzene. From this we may conclude that the nitration reagent is more reactive and less selective than the halogenation reagents.
Both sulfonation and nitration yield h2o equally a by-product. This does not significantly affect the nitration reaction (note the presence of sulfuric acid every bit a dehydrating agent), simply sulfonation is reversible and is driven to completion by improver of sulfur trioxide, which converts the h2o to sulfuric acid. The reversibility of the sulfonation reaction is occasionally useful for removing this functional grouping.
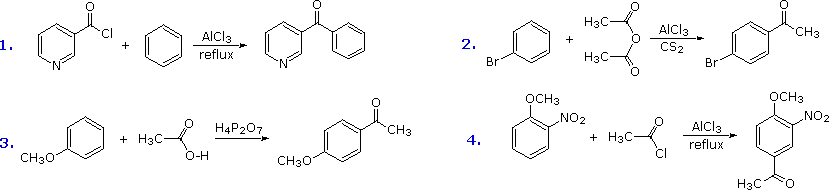
The Friedel-Crafts acylation reagent is normally composed of an acyl halide or anhydride mixed with a Lewis acid catalyst such equally AlCliii. This produces an acylium cation, R-C≡O(+), or a related species. Such electrophiles are not exceptionally reactive, and then the acylation reaction is mostly restricted to aromatic systems that are at least every bit reactive equally chlorobenzene. Carbon disulfide is often used as a solvent, since it is unreactive and is easily removed from the product. If the substrate is a very reactive benzene derivative, such as anisole, carboxylic esters or acids may be the source of the acylating electrophile. Some examples of Friedel-Crafts acylation reactions are shown in the following diagram. The kickoff demonstrates that unusual acylating agents may be used as reactants. The 2d makes utilise of an anhydride acylating reagent, and the third illustrates the ease with which anisole reacts, as noted earlier. The HfourPiiO7 reagent used hither is an anhydride of phosphoric acrid called pyrophosphoric acrid. Finally, the fourth example illustrates several important points. Since the nitro grouping is a powerful deactivating substituent, Friedel-Crafts acylation of nitrobenzene does not take place nether any conditions. However, the presence of a 2d strongly-activating substituent grouping permits acylation; the site of reaction is that favored by both substituents.
A mutual characteristic of the halogenation, nitration, sulfonation and acylation reactions is that they introduce a deactivating substituent on the benzene ring. Equally a result, we practice not commonly have to worry about disubstitution products existence formed. Friedel-Crafts alkylation, on the other hand, introduces an activating substituent (an alkyl group), and then more than one commutation may accept place. If benzene is to be alkylated, as in the post-obit synthesis of tert-butylbenzene, the mono-alkylated product is favored past using a big excess of this reactant. When the molar ratio of benzene to alkyl halide falls below ane:1, para-ditert-butylbenzene becomes the major product.
CviH6 (big backlog) + (CH3)3C-Cl + AlCl3 —— > C6H5-C(CH3)3 + HCl
The carbocation electrophiles required for alkylation may exist generated from alkyl halides (as above), alkenes + strong acid or alcohols + strong acid. Since 1º-carbocations are prone to rearrangement, it is usually not possible to introduce 1º-alkyl substituents larger than ethyl by Friedel-Crafts alkylation. For example, reaction of excess benzene with ane-chloropropane and aluminum chloride gives a good yield of isopropylbenzene (cumene).
Boosted examples of Friedel-Crafts alkylation reactions are shown in the following diagram.
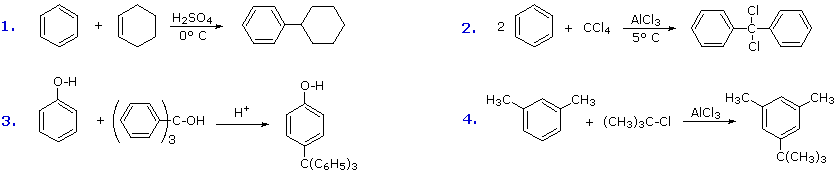
The first and third examples testify how alkenes and alcohols may be the source of the electrophilic carbocation reactant. The triphenylmethyl cation generated in the third instance is relatively unreactive, due to extensive resonance charge delocalization, and only substitutes highly activated aromatic rings. The 2nd example shows an interesting case in which a polychlororeactant is used equally the alkylating agent. A four fold backlog of carbon tetrachloride is used to avert tri-alkylation of this reagent, a process that is retarded past steric hindrance. The fourth case illustrates the poor orientational selectivity oft constitute in alkylation reactions of activated benzene rings. The bulky tert-butyl group ends upward attached to the reactive meta-xylene ring at the to the lowest degree hindered site. This may non exist the site of initial bonding, since polyalkylbenzenes rearrange nether Friedel-Crafts conditions (para-dipropylbenzene rearranges to meta-dipropylbenzene on heating with AlCl3).
A practical business in the use of electrophilic effluvious substitution reactions in synthesis is the separation of isomer mixtures. This is peculiarly truthful for cases of ortho-para substitution, which often produce significant amounts of the pocket-sized isomer. Every bit a dominion, para-isomers predominate except for some reactions of toluene and related alkyl benzenes. Separation of these mixtures is aided by the fact that para-isomers have significantly higher melting points than their ortho counterparts; consequently, fractional crystallization is often an effective isolation technique. Since meta-substitution favors a unmarried product, separation of trace isomers is normally not a trouble.
four. Electrophilic Substitution of Disubstituted Benzene Rings
When a benzene ring has two substituent groups, each exerts an influence on subsequent substitution reactions. The activation or deactivation of the band tin be predicted more or less past the sum of the individual effects of these substituents. The site at which a new substituent is introduced depends on the orientation of the existing groups and their individual directing effects. We tin can identify 2 general behavior categories, equally shown in the following table. Thus, the groups may be oriented in such a manner that their directing influences act in concert, reinforcing the effect; or are opposed (antagonistic) to each other. Note that the orientations in each category change depending on whether the groups have similar or opposite individual directing effects.
Orientational Interaction of Substituents | ||
|---|---|---|
| Combative or Non-Cooperative | Reinforcing or Cooperative | |
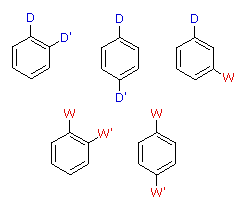 | 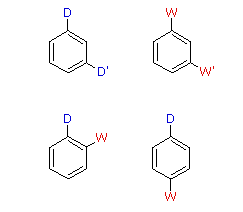 | |
| D = Electron Donating Grouping (ortho/para-directing) | ||
The products from exchange reactions of compounds having a reinforcing orientation of substituents are easier to predict than those having antagonistic substituents. For example, the 6 equations shown below are all examples of reinforcing or cooperative directing effects operating in the expected fashion. Symmetry, every bit in the showtime two cases, makes information technology piece of cake to predict the site at which substitution is likely to occur. Note that if two different sites are favored, substitution will usually occur at the 1 that is least hindered by ortho groups.
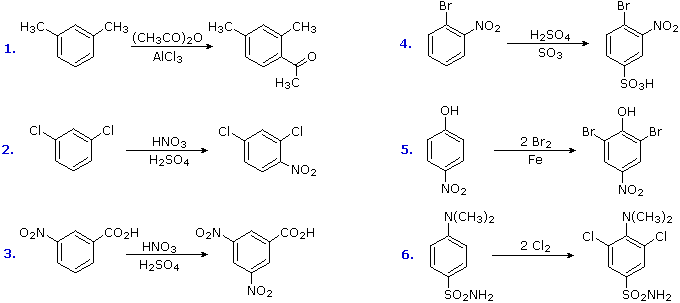
The first three examples have two like directing groups in a meta-relationship to each other. In examples iv through 6, oppositely directing groups accept an ortho or para-relationship. The major products of electrophilic substitution, as shown, are the sum of the private grouping furnishings. The strongly activating hydroxyl (–OH) and amino (–NH2) substituents favor dihalogenation in examples 5 and six.
Substitution reactions of compounds having an antagonistic orientation of substituents require a more careful assay. If the substituents are identical, every bit in example 1 below, the symmetry of the molecule will again simplify the conclusion. When one substituent has a pair of not-bonding electrons bachelor for adjacent charge stabilization, it will normally exert the production determining influence, examples 2, 4 & v, even though information technology may be overall deactivating (case ii). Case 3 reflects a combination of steric hindrance and the superior innate stabilizing ability of methyl groups relative to other alkyl substituents. Example 6 is interesting in that it demonstrates the conversion of an activating ortho/para-directing group into a deactivating meta-directing "onium" cation [–NH(CHiii)2 (+) ] in a strong acid environs.
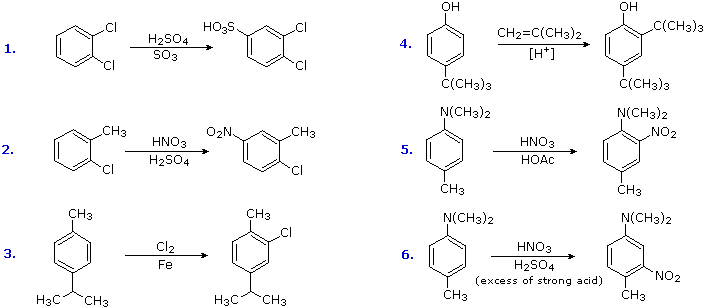
![]()
Reactions of Substituent Groups
1. Oxidation of Alkyl Side-Chains
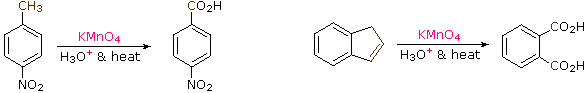
The benzylic hydrogens of alkyl substituents on a benzene band are activated toward gratis radical attack, as noted earlier. Furthermore, SN1, SN2 and E1 reactions of benzylic halides, bear witness enhanced reactivity, due to the next aromatic ring. The possibility that these observations reverberate a general benzylic activation is supported by the susceptability of alkyl side-chains to oxidative deposition, as shown in the following examples (the oxidized side chain is colored). Such oxidations are usually effected past hot acidic pemanganate solutions, but for large scale industrial operations catalysed air-oxidations are preferred. Interstingly, if the benzylic position is completely substituted this oxidative degradation does non occur (2nd equation, the substituted benzylic carbon is colored blue).
These equations are non balanced. The permanganate oxidant is reduced, usually to Mn(IV) or Mn(Ii). Two other examples of this reaction are given below, and illustrate its usefulness in preparing substituted benzoic acids.
2. Reduction of Nitro Groups and Aryl Ketones
Electrophilic nitration and Friedel-Crafts acylation reactions innovate deactivating, meta-directing substituents on an aromatic ring. The attached atoms are in a high oxidation country, and their reduction converts these electron withdrawing functions into electron altruistic amino and alkyl groups. Reduction is easily achieved either past catalytic hydrogenation (H2 + goad), or with reducing metals in acid. Examples of these reductions are shown hither, equation 6 demonstrating the simultaneous reduction of both functions. Note that the butylbenzene product in equation iv cannot be generated by direct Friedel-Crafts alkylation due to carbocation rearrangement. The zinc used in ketone reductions, such as v, is usually activated by alloying with mercury (a procedure known as amalgamation).
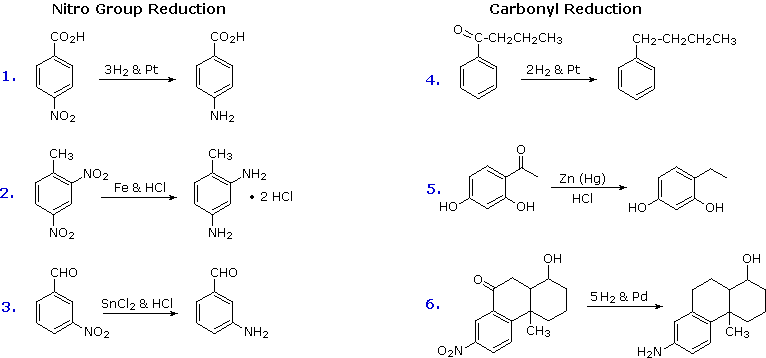
Several culling methods for reducing nitro groups to amines are known. These include zinc or tin in dilute mineral acid, and sodium sulfide in ammonium hydroxide solution. The procedures described above are sufficient for most cases.
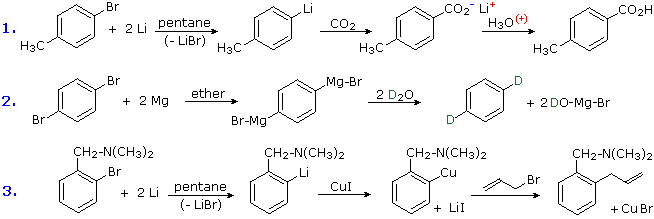
3. Conversion of Halogens to Organometallic Reagents
The reaction of alkyl and aryl halides with reactive metals (usually Li & Mg) to requite nucleophilic reagents has been noted. This provides a powerful tool for the conversion of chloro, bromo or iodo substituents into a variety of other groups. Many reactions of these aryl lithium and Grignard reagents volition be discussed in afterwards sections, and the following equations provide typical examples of carboxylation, protonation and Gilman coupling. Metal element of group vii exchange reactions take place at depression temperature, and may exist used to introduce iodine at designated locations. An example of this method will be displayed beneath past clicking on the diagram. In this example care must be taken to maintain a low temperature, because elimination to an aryne intermediate takes place on warming.
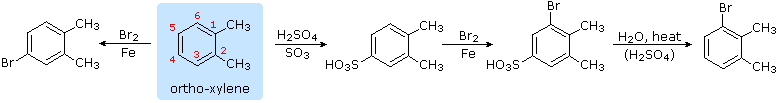
4. Hydrolysis of Sulfonic Acids
The potential reversibility of the aromatic sulfonation reaction was noted earlier. The following equation illustrates how this characteristic of the sulfonic acids may be used to prepare the three-bromo derivative of ortho-xylene. Direct bromination would requite the 4-bromo derivative.
5. Modifying the Influence of Potent Activating Groups
The strongest activating and ortho/para-directing substituents are the amino (-NH2) and hydroxyl (-OH) groups. Directly nitration of phenol (hydroxybenzene) by dilute nitric acrid gives modest yields of nitrated phenols and considerable oxidative decomposition to tarry materials; aniline (aminobenzene) is largely destroyed. Bromination of both phenol and aniline is difficult to command, with di- and tri-bromo products forming readily. Because of their high nucleophilic reactivity, aniline and phenol undergo substitution reactions with iodine, a element of group vii that is usually unreactive with benzene derivatives. The mixed halogen iodine chloride (ICl) provides a more electrophilic iodine moiety, and is effective in iodinating aromatic rings having less powerful activating substituents.
| Chalf-dozenH5–NH2 + Itwo + NaHCOthree |  | p-I–C6H4–NH2 + NaI + CO2 + H2O |
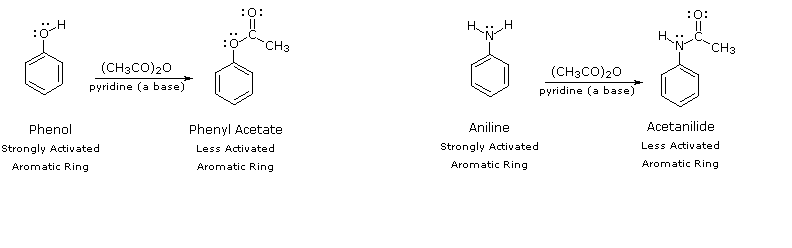
By acetylating the heteroatom substituent on phenol and aniline, its activating influence tin can be essentially attenuated. For example, acetylation of aniline gives acetanilide (get-go stride in the following equation), which undergoes nitration at low temperature, yielding the para-nitro product in loftier yield. The modifying acetyl group tin then exist removed by acid-catalyzed hydrolysis (last stride), to yield para-nitroaniline. Although the activating influence of the amino group has been reduced past this procedure, the acetyl derivative remains an ortho/para-directing and activating substituent.
| C6H5–NH2 + (CHthreeCO)2O | pyridine (a base) | C6H5–NHCOCHiii | HNOthree , 5 ºC | p-OtwoN–Chalf dozenH4–NHCOCHiii | H3O(+) & heat | p-O2North–Chalf dozenHiv–NH2 |
The following diagram illustrates how the acetyl group acts to attenuate the overall electron donating character of oxygen and nitrogen. The non-bonding valence electron pairs that are responsible for the high reactivity of these compounds (bluish arrows) are diverted to the next carbonyl group (green arrows). However, the overall influence of the modified substituent is still activating and ortho/para-directing.
 |
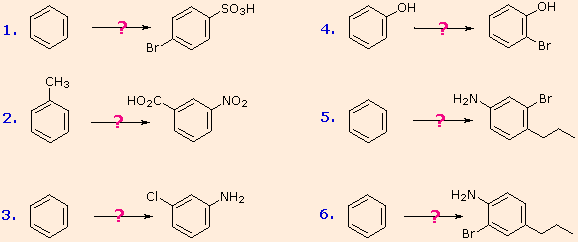
It should now be apparent that an extensive "toolchest" of reactions are available to u.s.a. for the synthesis of substituted benzenes. Just as an expert carpenter must sympathise the characteristics and limitations of his/her tools, chemists must appreciate the nature of their "tools" when applying them to a specific synthesis. Six proposed syntheses are listed in the following diagram in crude guild of increasing complexity. You should try to excogitate a plausible reaction sequence for each. Once y'all have done so, yous may check suggested answers past clicking on the question mark for each.
 |
Reactions of Fused Benzene Rings
Compounds in which two or more than benzene rings are fused together were described in an earlier chapter, and they nowadays interesting insights into aromaticity and reactivity. The smallest such hydrocarbon is naphthalene. Naphthalene is stabilized past resonance. Three canonical resonance contributors may be drawn, and are displayed in the following diagram.
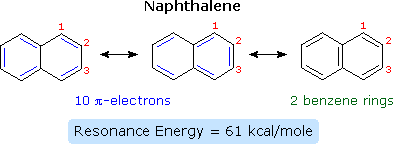
The two structures on the left take one detached benzene ring each, but may also exist viewed as x-pi-electron annulenes having a bridging single bond. The structure on the correct has two benzene rings which share a common double bond. From heats of hydrogenation or combustion, the resonance energy of naphthalene is calculated to be 61 kcal/mole, 11 kcal/mole less than that of 2 benzene rings (ii * 36). Every bit expected from an average of the three resonance contributors, the carbon-carbon bonds in naphthalene show variation in length, suggesting some localization of the double bonds. The C1–C2 bond is i.36 Å long, whereas the C2–C3 bond length is 1.42 Å. This contrasts with the construction of benzene, in which all the C–C bonds have a common length, 1.39 Å.
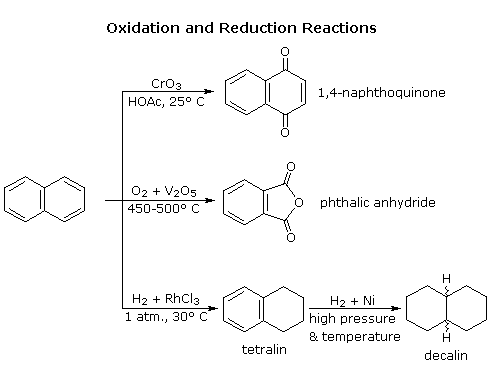
Naphthalene is more reactive than benzene, both in commutation and addition reactions, and these reactions tend to keep in a mode that maintains one intact benzene ring. The following diagram shows 3 oxidation and reduction reactions that illustrate this feature. In the last example, catalytic hydrogenation of i ring takes place under milder weather condition than those required for complete saturation (the decalin product exists as cis/trans isomers). Electrophilic substitution reactions take place more than chop-chop at C1, although the C2 product is more stable and predominates at equilibrium. Examples of these reactions will exist displayed past clicking on the diagram. The kinetically favored C1 orientation reflects a preference for generating a cationic intermediate that maintains one intact benzene ring. By clicking on the diagram a second time, the two naphthenonium intermediates created by attack at C1 and C2 will be displayed.
The construction and chemistry of more highly fused benzene ring compounds, such as anthracene and phenanthrene show many of the same characteristics described above.
Nucleophilic Substitution, Elimination & Addition Reactions of Benzene Derivatives
ane. Commutation
An early method of preparing phenol (the Dow process) involved the reaction of chlorobenzene with a concentrated sodium hydroxide solution at temperatures above 350 ºC. The chief products are phenol and diphenyl ether (see below). This credible nucleophilic substitution reaction is surprising, since aryl halides are more often than not incapable of reacting by either an SouthwardN1 or SN2 pathway.
| C6Hv–Cl + NaOH solution | 350 ºC | C6H5–OH + C6H5–O–Chalf-dozenH5 + NaCl |
The presence of electron-withdrawing groups (such as nitro) ortho and para to the chlorine substancially enhance the rate of substitution, as shown in the set of equations presented on the left below. To explain this, a tertiary mechanism for nucleophilic substitution has been proposed. This two-step mechanism is characterized by initial addition of the nucleophile (hydroxide ion or water) to the aromatic ring, followed by loss of a halide anion from the negatively charged intermediate. This is illustrated by clicking the "Show Machinery" button next to the diagram. The sites over which the negative accuse is delocalized are colored bluish, and the ability of nitro, and other electron withdrawing, groups to stabilize next negative accuse accounts for their charge per unit enhancing influence at the ortho and para locations.
Iii additional examples of aryl halide nucleophilic substitution are presented on the right. Just the two- and iv-chloropyridine isomers undergo rapid commutation, the 3-chloro isomer is relatively unreactive. Nitrogen nucleophiles volition also react, as evidenced by the utilise of Sanger'southward reagent for the derivatization of amino acids. The resulting Northward-2,4-dinitrophenyl derivatives are bright yellow crystalline compounds that facilitated assay of peptides and proteins, a bailiwick for which Frederick Sanger received one of his two Nobel Prizes in chemistry.
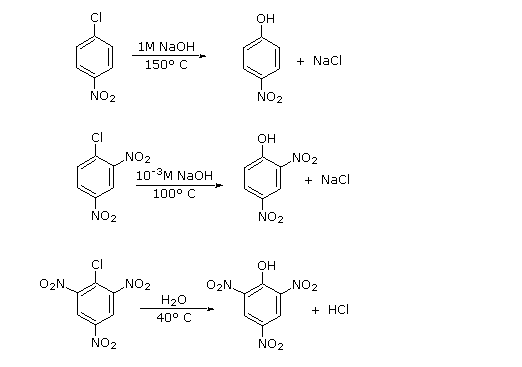 | Boosted Examples
|
|---|
Such addition-elimination processes generally occur at sp2 or sp hybridized carbon atoms, in dissimilarity to SNorth1 and SouthwardN2 reactions. When practical to aromatic halides, every bit in the present discussion, this mechanism is called SNAr. Some distinguishing features of the three common nucleophilic exchange mechanisms are summarized in the post-obit table.
| Mechanism | Number of Steps | Bond Formation Timing | Carbon Hybridization |
|---|---|---|---|
| SouthN1 | Ii | After Bond Breaking | Usually sp3 |
| Due southNorth2 | One | Simultaneous with | Usually sp3 |
| SNorthAr | 2 | Prior to Bond Breaking | Usually sp2 |
2. Emptying
At that place is skilful bear witness that the synthesis of phenol from chlorobenzene does not keep by the addition-elimination machinery (SouthNAr) described above. For example, treatment of para-chlorotoluene with sodium hydroxide solution at temperatures higher up 350 ºC gave an equimolar mixture of meta- and para-cresols (hydroxytoluenes). Chloro and bromobenzene reacted with the very potent base sodium amide (NaNH2 at depression temperature (-33 ºC in liquid ammonia) to give good yields of aniline (aminobenzene). However, ortho-chloroanisole gave exclusively meta-methoxyaniline under the same conditions. These reactions are described by the following equations.
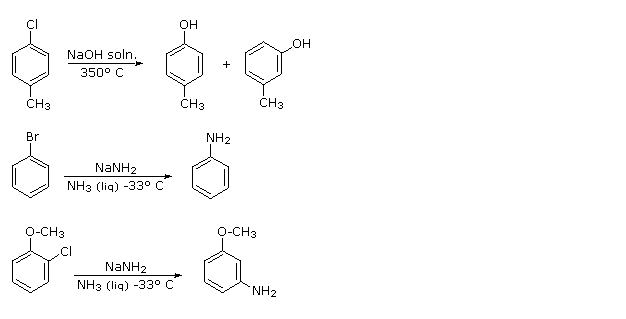 |
The explanation for this curious repositioning of the substituent grouping lies in a different two-step mechanism we tin can refer to equally an elimination-addition process. The intermediate in this machinery is an unstable benzyne species, as displayed in the in a higher place illustration by clicking the "Show Mechanism" button. In contrast to the parallel overlap of p-orbitals in a stable alkyne triple bond, the p-orbitals of a benzyne are tilted ca.120º apart, and so the reactivity of this incipient triple bond to addition reactions is profoundly enhanced. In the absence of steric hindrance (top instance) equal amounts of meta- and para-cresols are obtained. The steric bulk of the methoxy group and the ability of its ether oxygen to stabilize an adjacent anion result in a substantial bias in the add-on of amide anion or ammonia.
For boosted information well-nigh benzyne and related species , Click Here.
3. Addition
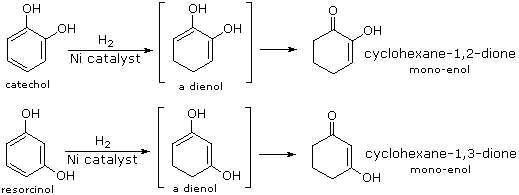
Although it does so less readily than unproblematic alkenes or dienes, benzene adds hydrogen at high pressure in the presence of Pt, Pd or Ni catalysts. The production is cyclohexane and the heat of reaction provides evidence of benzene's thermodynamic stability. Substituted benzene rings may also be deduced in this manner, and hydroxy-substituted compounds, such every bit phenol, catechol and resorcinol, requite carbonyl products resulting from the fast ketonization of intermediate enols. Nickel catalysts are frequently used for this purpose, as noted in the following equations.
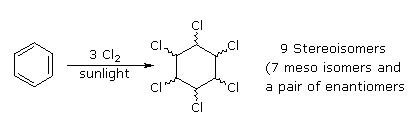
Benzene is more susceptible to radical add-on reactions than to electrophilic addition. We take already noted that benzene does not react with chlorine or bromine in the absence of a catalyst and rut. In strong sunlight or with radical initiators benzene adds these halogens to give hexahalocyclohexanes. It is worth noting that these same atmospheric condition effect radical commutation of cyclohexane, the key factors in this change of behavior are the pi-bonds assortment in benzene, which permit addition, and the weaker C-H bonds in cyclohexane. The add-on of chlorine is shown beneath; two of the seven meso-stereoisomers will appear if the "Show Isomer" button is clicked.
 |
The Birch Reduction
Another way of adding hydrogen to the benzene band is past treatment with the electron rich solution of brine metals, usually lithium or sodium, in liquid ammonia. To see examples of this reaction, which is called the Birch Reduction, Click Hither.
| Practice Problems |
|---|
| The following bug review various aspects of effluvious chemical science. The first two questions review some unproblematic concepts. The side by side two questions require you to analyze the directing influence of substituents. The fifth question asks you lot to draw the products of some aromatic commutation reactions. The sixth question takes you through a mutistep synthesis. The terminal pick leads to a large number of multiple selection questions. |
Phenols
Reactions of Phenols
Compounds in which a hydroxyl group is bonded to an aromatic ring are called phenols. The chemical behavior of phenols is different in some respects from that of the alcohols, and then information technology is sensible to care for them every bit a like but characteristically distinct grouping. A corresponding divergence in reactivity was observed in comparison aryl halides, such as bromobenzene, with alkyl halides, such equally butyl bromide and tert-butyl chloride. Thus, nucleophilic commutation and elimination reactions were mutual for alkyl halides, merely rare with aryl halides. This distinction carries over when comparing alcohols and phenols, so for all practical purposes substitution and/or elimination of the phenolic hydroxyl group does not occur.
1. Acidity of Phenols
On the other manus, exchange of the hydroxyl hydrogen atom is even more facile with phenols, which are roughly a million times more acidic than equivalent alcohols. This phenolic acidity is further enhanced by electron-withdrawing substituents ortho and para to the hydroxyl grouping, as displayed in the following diagram. The alcohol cyclohexanol is shown for reference at the pinnacle left. It is noteworthy that the influence of a nitro substituent is over ten times stronger in the para-location than it is meta, despite the fact that the latter position is closer to the hydroxyl group. Furthermore additional nitro groups take an additive influence if they are positioned in ortho or para locations. The trinitro compound shown at the lower right is a very strong acid called picric acid.
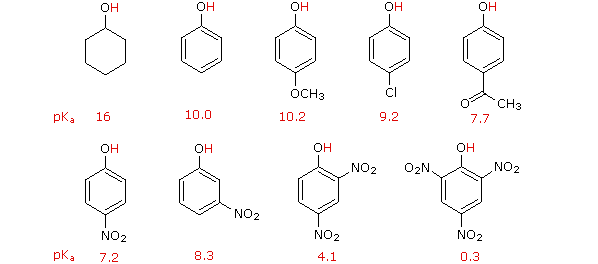
Why is phenol a much stronger acrid than cyclohexanol? To reply this question nosotros must evaluate the fashion in which an oxygen substituent interacts with the benzene ring. Equally noted in our before handling of electrophilic aromatic substitution reactions, an oxygen substituent enhances the reactivity of the ring and favors electrophile assault at ortho and para sites. It was proposed that resonance delocalization of an oxygen non-bonded electron pair into the pi-electron system of the aromatic ring was responsible for this substituent effect. Formulas illustrating this electron delocalization will be displayed when the "Resonance Structures" button beneath the previous diagram is clicked. A like prepare of resonance structures for the phenolate anion conjugate base appears beneath the phenol structures. 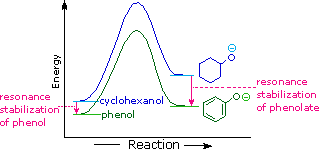
The resonance stabilization in these two cases is very different. An important principle of resonance is that charge separation diminishes the importance of canonical contributors to the resonance hybrid and reduces the overall stabilization. The contributing structures to the phenol hybrid all suffer charge separation, resulting in very small stabilization of this chemical compound. On the other mitt, the phenolate anion is already charged, and the canonical contributors act to disperse the charge, resulting in a substantial stabilization of this species. The cohabit bases of simple alcohols are not stabilized by accuse delocalization, so the acerbity of these compounds is similar to that of water. An energy diagram showing the effect of resonance on cyclohexanol and phenol acidities is shown on the right. Since the resonance stabilization of the phenolate conjugate base is much greater than the stabilization of phenol itself, the acerbity of phenol relative to cyclohexanol is increased. Supporting evidence that the phenolate negative charge is delocalized on the ortho and para carbons of the benzene ring comes from the influence of electron-withdrawing substituents at those sites. The additional resonance stabilization provided by ortho and para nitro substituents will be displayed past clicking the "Resonance Structures" button a 2d time. You may wheel through these illustrations by repeated clicking of the push.
2. Substitution of the Hydroxyl Hydrogen
Equally with the alcohols, the phenolic hydroxyl hydrogen is rather easily replaced by other substituents. For instance, phenol reacts easily with acerb anhydride to requite phenyl acetate. Likewise, the phenolate anion is an effective nucleophile in SNorth2 reactions, as in the 2d case below.
C6H5–OH + (CH3CO)iiO  C6Hv–O–COCHthree + CH3COtwoH
C6Hv–O–COCHthree + CH3COtwoH
Chalf-dozenH5–O(–) Na(+) + CH3CH2CH3–Br  Chalf dozenH5–O–CH2CHiiCHthree + NaBr
Chalf dozenH5–O–CH2CHiiCHthree + NaBr
iii. Electrophilic Substitution of the Aromatic Band
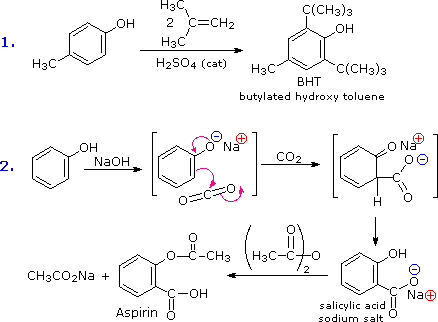
The facility with which the aromatic ring of phenols and phenol ethers undergoes electrophilic exchange has been noted. Two examples are shown in the following diagram. The showtime shows the Friedel-Crafts synthesis of the nutrient preservative BHT from para-cresol. The second reaction is interesting in that it further demonstrates the delocalization of charge that occurs in the phenolate anion. Carbon dioxide is a weak electrophile and ordinarily does not react with aromatic compounds; however, the negative charge concentration on the phenolate ring enables the carboxylation reaction shown in the 2d step. The sodium common salt of salicylic acid is the major ptoduct, and the preference for ortho substitution may reflect the influence of the sodium cation. This is called the Kolbe-Schmidt reaction, and it has served in the grooming of aspirin, as the last stride illustrates.
4. Oxidation of Phenols
Phenols are rather easily oxidized despite the absence of a hydrogen atom on the hydroxyl begetting carbon. Among the colored products from the oxidation of phenol by chromic acid is the dicarbonyl compound para-benzoquinone (also known as ane,4-benzoquinone or only quinone); an ortho isomer is likewise known. These compounds are easily reduced to their dihydroxybenzene analogs, and it is from these compounds that quinones are all-time prepared. Note that meta-quinones having similar structures practice non exist. The redox equilibria between the dihydroxybenzenes hydroquinone and catechol and their quinone oxidation states are and so facile that milder oxidants than chromate (Jones reagent) are generally preferred. I such oxidant is Fremy's salt, shown on the right. Reducing agents other than stannous chloride (eastward.1000. NaBH4) may exist used for the reverse reaction.
The position of the quinone-hydroquinone redox equilibrium is proportional to the foursquare of the hydrogen ion concentration, as shown by the following one-half-reactions (electrons are colored blueish). The electrode potential for this interconversion may therefore be used to mensurate the pH of solutions.
| Quinone + 2H(+) | 2e(–)  –2e(–) | Hydroquinone |
|---|
Although chromic acid oxidation of phenols having an unsubstituted para-position gives some p-quinone production, the reaction is complex and is not synthetically useful. It has been plant that salcomine, a cobalt circuitous, binds oxygen reversibly in solution, and catalyzes the oxidation of various substituted phenols to the respective p-quinones. The structure of salcomine and an example of this reaction are shown in the following equation. The solvent of choice for these oxidations is ordinarily methanol or dimethylformamide (DMF).
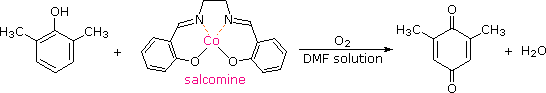
| Exercise Problems |
|---|
| The outset problem concerns the relative acidity of different functional groups. The concluding asks you to draw the product of a reaction selected from 48 possible combinations of alcohols or phenols and selected reagents. |
| Return to Table of Contents |
|---|
![]()
Bromination Of Acetanilide Balanced Equation,
Source: https://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/chapt15.htm
Posted by: yuenbegamseley.blogspot.com



0 Response to "Bromination Of Acetanilide Balanced Equation"
Post a Comment